Re: Chloramine and suggested treatment
Thanks Ron,
I'll now go back to my precious tome that NS scrapped in the wee hours of this morning. :wink: Of course I have forgotten all the good points I made in that one. :^)
Many US aquarists were at least as casual as you in SG about water treatment. "It always worked for me, before," became a sad lament that I heard all too often five or six years ago.
Much US water supply has such low bacteria counts that no chlorine is needed at all. That's neat!
Here around Bishop, only one well out of many in the area shows enough coliform bacteria to ever require chlorine. [Unfortunately, I don't know the exact part of town that it serves, so all I can say is "test." AFAIK, chloramines are not used here at all.]
My water is nearly perfect, containing enough Ca and Mg to sustain life (50 ppm) enough other minerals to be healthy (total dissolved solids 85-89 ppm) and higher than acceptable iron which stains the toilet scale but makes plants thrive (300 ppb). The big problem with iron is that it is a flavor amplifier, so can influence drinking water taste and cooking in some ways. I have no chlorine, but it could give an awful taste with that much iron present.
I can add a bit of salt to a Notho tank without poisoning them. Down town the 30 ppm water is too soft for life, and any added sodium tends to be lethal without adding potassium (aka "No Salt") for proper cell metabolism. My local well is a few units down and just across the driveway from my home. Not much happens there that I can't monitor. 
Historically, the testing for bacteria and use of chlorine in 1st-world countries eliminated the travelers' diseases and made the water safe for all tourists.
Mature water distribution systems always have some buildup of organic debris. When I was at Hewlett Packard's Corporate Research Labs we couldn't even keep it out of our 20 MegOhm DI water lines! Frustrating when trying to make state-of-the-art semiconductors!
Chlorine combines with those organics to produce a class of chemicals called trihalomethanes. A familiar one was once used as an anaesthetic -- chloroform. Unfortunately, they were found to cause cancer, so a solution was needed. First we need to back up a few years.
Years ago, folks in the US midwest found that agriculture runoff, containing ammonium from fertilizers (and cow pee?), combined with chlorine in the water to make a much more stable group of compounds called chloramines. Overnight aeration did not remove the compounds and they really killed or made fish sick. John F. Kuhns, of Missouri, investigated the problem and ended up patenting a compound, much like formaldehyde, that neutralized the chlorine just as well as the old photographer's hypo (sodium thiosulfate) but also tied up the ammonium. He licensed his patent to Novalek (Kordon) and they marketed it under their trade name of "Amquel." It was great as the ammonium was still available to the plants or biofilter, but didn't burn the gills by turning to ammonia.
This was particularly vital to the fish farms all down the Mississippi River, but ornamental aquaria got the benefits, too. When the Environmental Protection Agency discovered the problems with trihalomethanes, they looked around and found that aquaculture had been dealing with chloramines for years and that there was a lot of knowledge available.
In addition to stabilizing the chlorine so it wasn't gone in a day or two, converting it to chloramine stopped formation of trihalomethanes without slowing its antibacterial properties at all. Eureka! The best of all worlds (if you aren't a fish or shrimp).
EPA quickly mandated that all municipal water supplies, starting with the largest ones, should convert to chloramines from chlorine only. It was cheap and easy. Add some bleach and add some ammonium, and the water was safe, again.
Many older homes in the US used all copper pipes, with solder joints of lead/tin, and the etching of the heavy metals was detected in the blood of children exposed. By requiring the use of ammonium hydroxide to raise pH to 9 or 10 they stopped the heavy-metal toxins cold. [They may use lye (sodium hydroxide) in some places where ammonium isn't needed.] This mandate was, again, a very user friendly but fish-damaging change in the water treatment.
In my area, San Jose was the first district to switch to chloramines. I lived in the next town north, Santa Clara, and received hard well water with neither chlorine nor chloramine, normally. Most of the towns north of me had local wells but bought a lot of San Francisco water from the Hetch Hetchy reservoir in the Sierras. The latter water was very soft and had no additives of any kind, as it was bacteria free. [SF finally had to add chloramines, just starting this year.]
The elements were in place for three or four years of running catastrophe. No matter how they tried, San Jose's water got mixed with both our Santa Clara water and the Hetch Hetchy water in towns all up the peninsula.
Early massive fish losses in the local fish stores of San Jose were eventually handled (mostly by "Amquel"), but none of us were really prepared to deal with the problem in our own fishrooms.
During a drought in the mid '90s, Hetch Hetchy water was getting too turbid as they drew the water level closer to the mud. The solution was to send it to the big reservoirs around San Jose for settling, and then the water district ran it through huge RO filters in Santa Clara's industrial park to get the hardness back down where it was expected to be. That process wiped out a bunch of my fish, because they moved the water, very late at night, through a main that was about 1/4 mile from my house. It backed up into our well distribution system and I unknowingly changed water with heavily chloraminated San Jose water.
The city was grateful to me for pointing out the problem as that gave them another data point on where the contamination could spread. It did not, however, resuscitate a single one of my dead fish.
About that time, folks from Palo Alto to Belmont were reporting that their killies had suddenly become sterile. It turns out that the SF water processed from San Jose was being rechloraminated after the RO filtering and sent up the peninsula to the SF reservoirs at Crystal Springs. The level was mostly low and it didn't kill as much as it caused temporary sterility and eggs were going bad like crazy. We had one West Coast Weekend Show and Convention in Palo Alto, tho, where the mortality rate of fish exposed to local water was over 90%. It was carefully covered up, but I did the follow-up survey and know it happened. The show water was carefully blended and conditioned, but no one knew it was loaded with about 1 ppm of chloramines as it was San Jose water and not the Hetch Hetchy water usually available in Palo Alto.
Betta owners are prone to do 100% water changes, so misuse of chlorine removers was made very evident when chloramine showed up. The burst of ammonia released when the hypo "broke the chlorine-ammonium bond" was quite lethal when combined with the high pH that EPA was also mandating. There is 50X more deadly ammonia at pH 9 than at 7. At pH of 6.5 and below, it all stays as the relatively harmless ammonium ions (NH4+).
Two of the International Betta Congress's Grand Champions were totally wiped out when they failed to change the kind of conditioner they were using as their water was changed from chlorine to chloramine. Both of them lost many thousands of dollars worth of champion breeding stock as well as their next generation of young fish.
How do you handle this problem?
First of all, you are operating blind if you do not test. Chlorine tests are simple to use and very cheap at the pool and spa store. They are pretty costly at the LFS, but we do need to support those guys, so it is your call.
From the above narrative, you can see that predicting what the water service will do to you is too uncertain to risk your fish. Test, test, and test some more. Don't do any water additions or changes without checking for chlorine.
Don't test for ammonia as the kits are too insensitive (250 ppb least step vs 5 ppb damage threshold). The Nessler's reagent ones read positive for ammonia even if you use "Amquel" and they contain mercury which is a brute to dispose of safely. Salicylate tests are too slow and expensive and are no more sensitive than the others.
There is nothing wrong with using "Amquel" (or "Prime" or "Ammo Lock 2") to neutralize the bad effects of chloramine. BTW, "Amquel2" is not the same stuff, so avoid it. The primary downside is for active breeders who need to maintain infusoria cultures. I have even used "Amquel" to kill a hydra outbreak when I ran out of formaldehyde. 
They do kill small inverts and infusoria, so I prefer to use an in-line carbon block filter (known at your plumber's as a "Taste and Odor" filter) to strip the chloramine off of all incoming water before it ever gets to a tank.
Since Singapore is like Silicon Valley -- East, you can find great bargains at the industrial surplus houses, I'm sure. Carbon filters are heavily used in all kinds of high-tech manufacturing processes. While a new filter housing and cartridge might cost me US$35 at Home Depot, I could always find good used ones for a couple of dollars at the surplus places. Taste and Odor filter kits for ice-makers are about US$15, here, and include tubing and a saddle tap for the cold water pipe under your sink.
In a recent thread on killietalk, Lee Harper described building his own carbon filters for the flow-through water system he recently built. He made them so huge that they worked with the industrial carbon he could buy, so I will not say you cannot make a DIY filter system. I'm too cheap, and have thus always used surplus "bargains."
My experience with LFS carbon products has been nearly all negative, unfortunately. I'm sure a filter full of Ammo-Carb chips would be a lot better than nothing if you were silly enough to try to use an old-fashioned dechlorinator and released a massive dose of ammonia. IDK why one would do that, knowing that de-chlor products, like "Novaqua" are so deadly.
HTH
Wright
01 760 872-3995
805 Valley West Circle
Bishop, CA 93514 USA











 Reply With Quote
Reply With Quote
 and see who does maintenance
and see who does maintenance

 Is there still hope?? Whatever, Iíll have to try the new bottle to find out if it helps.
Is there still hope?? Whatever, Iíll have to try the new bottle to find out if it helps. 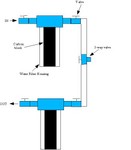


Bookmarks